FINAL EVENT of the Interreg 2 Seas project “Site-Specific Drug delivery”
on 30th March 2023 at 2:00-4:00pm (Brussels time)
live online (zoom) meeting
Participation is free of charge, but registration is required.
To register please send an email to alexandra.machado@univ-lille.fr, indicating your first and family name, position and institution.

PROGRAMME
Welcome
Parenteral, biodegradable drug delivery systems: Importance of the in vitro release set-up
Prof. Juergen Siepmann, University of Lille, France
Advanced drug delivery systems for colon targeting
Prof. Abdul Basit, University College London, UK
Innovative implants for inner ear treatments
Dr. Julian Apachitei, University of Delft, The Netherlands
Drug loaded breast implants
Dr. Julien Payen, Lattice Medical, Loos, France
Cutting edge in vitro characterization techniques for local drug delivery systems
Prof. Axel Zeitler, University of Cambridge, UK
Towards more realistic drug release measurements
Dr. Frédéric Moens, ProDigest, Belgium
Closing
The large majority of drug products available on the market deliver the drug in a non-targeted manner into the human body: The drug is circulated via the blood throughout the entire organism. Only parts of it reach the target site (e.g., a specific organ), while the large majority of the administered drug dose is distributed within the rest of the body. Importantly, this drug is not only “lost” for the treatment, it is often harmful for the patient, causing potentially severe side effects. The latter have 3 major consequences, they:
1) Constitute a major health burden for the patient (e.g. causing nausea, headache, cardiovascular complications).
2) Can limit the drug dose that can be given to the patient, leading to nonoptimal therapeutic effects (the drug amount reaching the target site cannot be further increased).
3) Cause considerable costs for the health care systems (e.g., due to the treatment of side effects, prolonged.
The aim of the “Site Drug” project is to develop innovative drug products, which are able to control the resulting drug distribution in the patient’s body: The drug amount at the site of action is optimized, and the amount that is “lost” into the rest of the human body is minimized. This is achieved by “site-specific delivery systems”, which release the drug at a controlled rate at the site of action. Thus, the therapeutic efficacy are improved & undesired side effects reduced. This will help reducing the current cost burden on our healthcare systems due to adverse drug effects.
Four types of disease and disorders are specifically addressed:
- Crohn’s disease and ulcerative colitis using colon targeting systems
- Ovarian cancer with metastases in the peritoneal cavity with biodegradable controlled drug delivery systems
- Breast resection due to cancer using biodegradable breast implants releasing drugs in a controlled manner
- Hearing loss/deafness using miniaturized implants.
A highly interdisciplinary and complementary consortium of 10 academic and industrial partners in France, Belgium, The Netherlands and the UK is jointly working on this project: Delft University of Technology; Ghent University; Lattice Medical; Leiden University; Lille University Hospital; Prodigest; Technological Transfer Office North of France; University College London; University of Cambridge; University of Lille.
Click below to download a pdf version of the flyer:
Dr Atheer Awad was recognised as one of International Pharmaceutical Federation’s (FIP) FIPWiSE Rising Stars

Dr Atheer Awad was recognised as one of International Pharmaceutical Federation’s (FIP) FIPWiSE Rising Stars.
FIPWiSE is FIP’s initiative for Women in Science and Education, which highlights women in pharmaceutical sciences or pharmacy education (including pharmaceutical practice research) who are pathfinders in their fields, who are rising in their careers and who deserve to be recognised.
Read our latests publications
3D printed multi-drug-loaded suppositories for acute severe ulcerative colitis
A. Awad, E. Hollis, A. Goyanes, M. Orlu, S. Gaisford, A. W. Basit
International Journal of Pharmaceutics: X, Volume 5, 2023, 100165
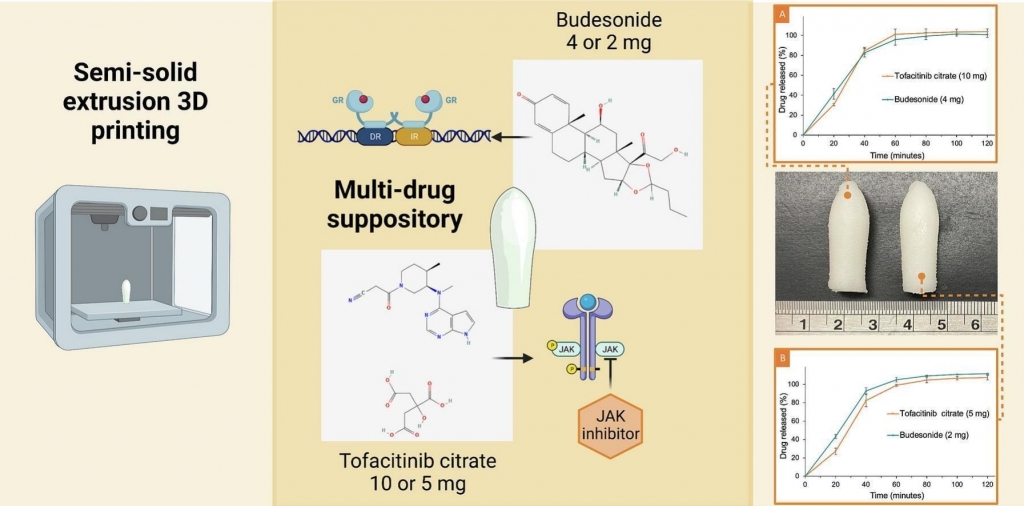
Acute severe ulcerative colitis (ASUC) is a growing health burden that often requires treatment with multiple therapeutic agents. As inflammation is localised in the rectum and colon, local drug delivery using suppositories could improve therapeutic outcomes. Three-dimensional (3D) printing is a novel manufacturing tool that permits the combination of multiple drugs in personalised dosage forms, created based on each patient’s disease condition. This study, for the first time, demonstrates the feasibility of producing 3D printed suppositories with two anti-inflammatory agents, budesonide and tofacitinib citrate, for the treatment of ASUC. As both drugs are poorly water-soluble, the suppositories’ ability to self-emulsify was exploited to improve their performance. The suppositories were fabricated via semi-solid extrusion (SSE) 3D printing and contained tofacitinib citrate and budesonide in varying doses (10 or 5 mg; 4 or 2 mg, respectively). The suppositories displayed similar dissolution and disintegration behaviours irrespective of their drug content, demonstrating the flexibility of the technology. Overall, this study demonstrates the feasibility of using SSE 3D printing to create multi-drug suppositories for the treatment of ASUC, with the possibility of titrating the drug doses based on the disease progression.
Reprinted from International Journal of Pharmaceutics: X, Volume 5, 2023, 100165, Awad et al., 3D printed multi-drug-loaded suppositories for acute severe ulcerative colitis, https://doi.org/10.1016/j.ijpx.2023.100165 under the Creative Commons CC BY NC ND License.
3D analysis of gerbil cochlea with cochlear implant
P. Toulemonde, M. Risoud, P.E. Lemesre, M. Tardivel, J. Siepmann, C. Vincent
European Annals of Otorhinolaryngology, Head and Neck Diseases
Volume 139, Issue 6, 2022, Pages 333-336
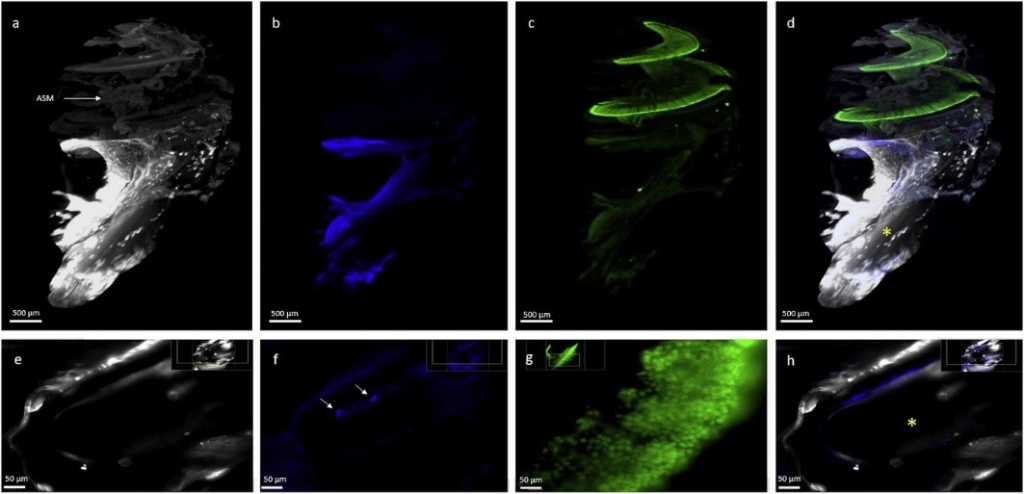
Objectives: To assess a clearing protocol using ethyl cinnamate, an organic substance which is non-toxic for humans, and its value in light-sheet microscopy study of post-implantation cochlear damage in the Mongolian gerbil.
Material and methods: The animals underwent right cochlear implantation in the round window by a retroauricular approach. They were then euthanized 10 weeks after implantation (electrode array in place). The cochleae were prepared according to a 29-day protocol including steps of fixation, microdissection, decalcification, permeabilization, blocking, fluorescent immunolabeling, dehydration and finally clearing in ethyl cinnamate solution. Acquisition of transparent cochleae was performed by light-sheet microscopy. Imaris software was then used for 3D analysis.
Results: The transparent cochleae had not undergone any shrinkage or any significant architectural changes. Six cochleae were acquired by light-sheet microscopy, allowing good visibility of the whole cochlea. 3D immunofluorescence analysis of the cochlea provided sufficient image resolution for analysis of the spiral ganglion neurons and assessment of the fibrotic tissue reaction surrounding the electrode array.
Conclusion: The ethyl cinnamate clearing protocol was effective for light-sheet microscopy analysis of the whole Mongolian gerbil cochlea with the implant left in situ. This technique is suitable for the study of post-implantation cell and tissue damage in the same sample, without the potential toxicity of other methods described to date.
Reprinted from Toulemonde et al., 3D analysis of gerbil cochlea with cochlear implant, European Annals of Otorhinolaryngology, Head and Neck Diseases, 2022, Volume 139 (6), 333-336. Copyright © 2022, Elsevier Masson SAS. All rights reserved.
Terahertz time-domain spectroscopy for powder compact porosity and pore shape measurements: An error analysis of the anisotropic bruggeman model
M. Anuschek, P. Bawuah, J. A. Zeitler
International Journal of Pharmaceutics: X, Volume 3, 2021, 100079
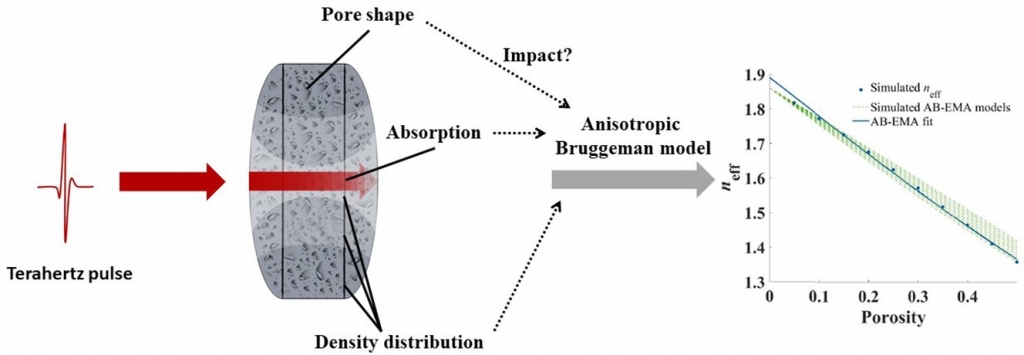
Terahertz time-domain spectroscopy (THz-TDS) is a novel technique which has been applied for pore structure analysis and porosity measurements. For this, mainly the anisotropic Bruggeman (AB-EMA) model is applied to correlate the effective refractive index (neff) of a tablet and the porosity as well as to evaluate the pore shape based on the depolarisation factor L. This paper investigates possible error sources of the AB-EMA for THz-TDS based tablet analysis. The effect of absorption and tablet anisotropy – changes of pore shape with porosity and density distribution – have been investigated. The results suggest that high tablet absorption has a negligible effect on the accuracy of the AB-EMA. In regards of tablet anisotropy the accuracy of the porosity determination is not impaired significantly. However, density distribution and variations in the pore shape with porosity resulted in an unreliable extraction of the tablet pore shape. As an extension of the AB-EMA a new concept was introduced to convert the model into bounds for L. This new approach was found useful to investigate tablet pore shape but also the applicability of the AB-EMA for an unknown set of data.
Reprinted from International Journal of Pharmaceutics: X, Volume 3, 2021, 100079, Anuschek et al. Terahertz time-domain spectroscopy for powder compact porosity and pore shape measurements: An error analysis of the anisotropic bruggeman model, https://doi.org/10.1016/j.ijpx.2021.100079 under the Creative Commons CC BY license.
